Market Analysis of Global Medical Informatization Industry in 2018
Overview of medical information in the United States
The medical informatization in the United States began with the new mission of the National Committee on Life and Health (NCVHS) in 1996 to provide medical information standardization. From 1996 to 2004, the medical informatization in the United States was in the exploratory stage; the fundamental turning point was in 2004. On April 27th, President Bush issued Presidential Decree No. 13335, which explicitly required electronic medical records to be implemented in the United States within 10 years. In 2009, President Obama issued Presidential Decree No. 13507 and issued the HITECH Act (“Health Information Technology for Economic and Clinical Healthâ€. The Act), medical informationization as part of the US healthcare reform; in 2010, the "Patient Protection and Affordable Care Act" was re-signed, followed by the "Federal Health Insurance and Federal Medical Assistance HER Award Scheme"; launched nationwide in 2015 Informatization construction of medical services; in 2016, the development of local standards for medical informatization in the United States was completed.
Figure 1: Schematic diagram of the development of medical informatization in the United States
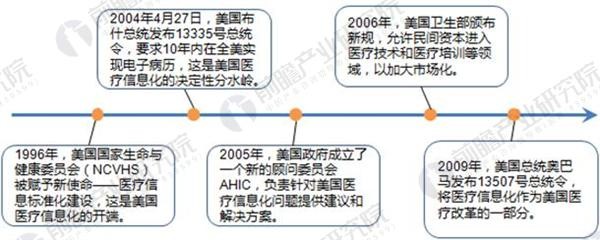
Source: Prospective Industry Research Institute
From the current state of technology of medical informatization, the United States is vigorously developing new medical information technology. In recent years, Google has partnered with medical centers in the United States to create electronic archives for millions of community patients, and doctors can remotely monitor them. Microsoft has also launched a new medical information service platform to help doctors, patients and patients' families know in real time. The latest state of the patient; Intel has launched a digital medical platform to help doctors interact with patients through IT; IBM has also made great efforts in this regard.
Overview of medical information in Japan
Japanese medical informationization began in the 1960s, and official medical informationization began in 1995. In the whole process of medical informationization in Japan, it can be seen that the development of medical informatization in Japan has received strong support from the government, and the government actively organizes research and development. At present, Japan is building electronic medical records from small clinics to large hospitals.
Figure 2: Schematic diagram of the development of medical informatization in Japan

Source: Prospective Industry Research Institute
Japan will focus on the development of electronic health records and telemedicine . Through e-health records, individuals can submit health information obtained by medical institutions to medical staff, thereby reducing the probability of misdiagnosis; at the same time, avoiding unnecessary inspections based on historical diagnostic records; and electronic delivery through prescriptions and electronic dispensing information It can provide follow-up feedback on prescription information or dispensing information, enabling safer, more convenient and high quality medical services.
Figure 3: List of the top five features of the Japanese electronic medical record file
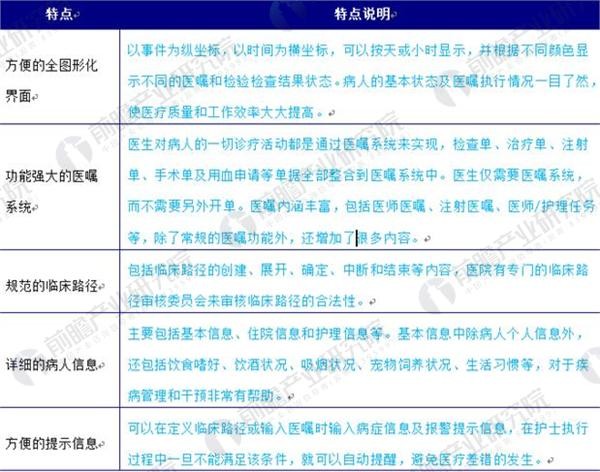
Source: Prospective Industry Research Institute
On the other hand, in response to medical problems such as shortage of doctors in some regions, Japan has promoted regional medical institutions to provide high-quality medical services to patients in remote areas through telemedicine programs. At the same time, the Japanese government has increased the construction of digital infrastructure for medical institutions, making diagnosis more efficient, thereby reducing the burden on medical workers and improving the management of hospitals.
As of 2017, the electronic medical record penetration rate of hospitals above designated size in Japan has reached 80%, but the disadvantage is that the data formats of different medical institutions and organizations are not unified with relevant medical standards, and it is impossible to conduct joint analysis of medical data in various fields. With such huge data, and with the development of artificial intelligence, Japan is ready to apply AI to support the development of health care.
Overview of European medical information
In recent years, the European medical informatization strategy has made great strides around these themes: electronic medical records, communication architecture and networks, standardization, security and privacy. However, due to other considerations, although some European countries (Sweden, Norway, Germany, Denmark, France, Iceland, Luxembourg, the United Kingdom) have decided to use medical information as a national strategy in the field of health, other European countries are still in central government coordination. The Ministry of Health and the Ministry of Civil Affairs and other relevant departments guide the policy formulation stage.
Figure 4: The structure of medical informatization in European countries
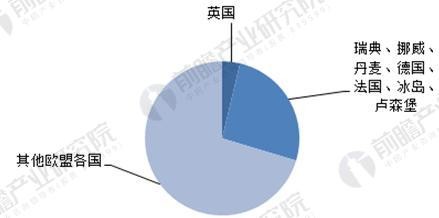
Source: Prospective Industry Research Institute
In the UK, more than 90% of doctors use computers, and 98% of the software used by doctors is mainly used to register patients, 94% for repeated prescriptions, and 29% for all clinical records. 14% achieved paperless office in the office. This laid a good IT foundation for the realization of telemedicine. As of 2017, the UK medical system has completed the construction of information technology, and the national health data are all networked and managed by the state, with high data security.
In Germany, the telemedicine system in Germany has entered the universal stage since the beginning of the 21st century. In recent years, cooperation between hospitals and communities in various health systems has been strengthened through telemedicine networks.
Taken together, the main features of the development of medical informatization in developed countries are shown in the table below.
Figure 5: List of main features of medical information development in developed countries

Source: Prospective Industry Research Institute
The above data and analysis are all from the Prospective Industry Research Institute's "2018-2023 China Medical Informatization Industry Market Preview and Investment Strategic Planning Analysis Report".
Microscope Slides,Microscope Glass Slides,Glass Microscope Slides,Frosted Microscope Slides
Yancheng Rongtai Labware Co.,Ltd , https://www.rongtailab.com
