FDA approves 23andMe's first consumer-oriented genetic testing product
In the US local time, on April 6, the FDA approved the personal genetic testing (GHR) developed by 23andMe for the risk of 10 diseases. The FDA said that this is the world's first FDA-approved direct sales. Genetic diagnosis products (services) for consumers (patients) can be based on the customer's genetic sequencing data, giving advice on health issues such as disease diagnosis and lifestyle.

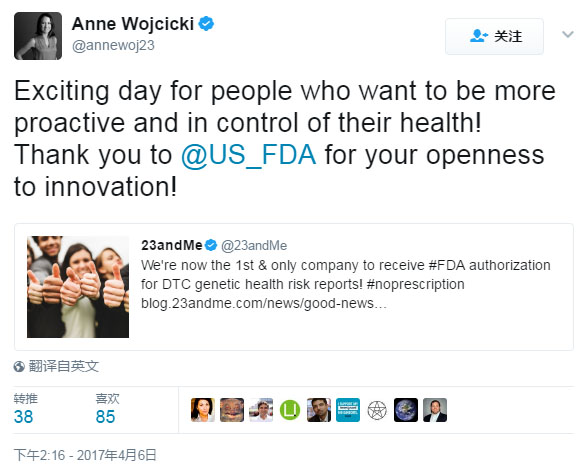
This is undoubtedly a positive for 23andMe, which suffers from policy and legality. Anne Wojcicki, CEO of 23andMe, said on Twitte on the same day, "This is a happy day for those who want more self-control of health, thanks to the FDA's open innovation," and said. We are now the first and only company to receive FDA approval for this service.
Since 2006, 23andMe has provided consumers with genetic testing services including 240 health risk assessments through saliva DNA testing, but in 2013 the test was stopped by the FDA. Two years later, although the FDA relaxed these restrictions, it has been plagued by policies and cannot provide similar services on a large scale.
On the same day, relevant news was also posted on the official Weibo of 23andMe.

Tuya Video Doorbell,Smart Doorbell Night Vision,Night Vision Doorbell,Camera Wireless Doorbell
Shenzhen Zuomi Technology Co., Ltd. , https://www.zuomicamera.com
