Digital pain management solves over-medical and drug abuse, Samsung and Philips have intervened, and the domestic market is almost blank
Pain is one of the most common symptoms in the clinic. It is the fifth vital sign after breathing, pulse, blood pressure and body temperature, which seriously affects the quality of life of patients. According to Wikipedia, pain management or pain management (pain management, pain control, pain control or algiatry) is an interdisciplinary approach to reducing pain and improving the quality of life of patients with chronic pain. Small branch.
According to data from WebMD's article on pain clinics, at least 100 million Americans and more than 1.5 billion people worldwide suffer from chronic pain. This number may be exaggerated, but the pain problem has become a hot issue of increasing concern.
This year, due to the increasing abuse of opioid abuse in the United States, the FDA first introduced a policy on opioid abuse in June this year, and then released digital innovations to stimulate pain management medical devices in less than a month.
In a segment that has not been valued in the past, there have been a series of innovative solutions due to drug abuse problems. According to the disclosure of events by Mobihealthnews and Medcitynews, such as the WellBrain platform acquisition incident, the FDA recently approved SPR Therapeutics pain management nerve stimulation wearable devices and other events caused the industry's attention, the arterial network has organized this, and accordingly reflect on the domestic market status .
Digital pain management innovation background
1. The spread of opioid therapy in the United States
The pain management drug market can be subdivided into narcotic analgesics, anticonvulsants, migraine drugs, antidepressants, opioids, and non-narcotic analgesics.
According to indications, pain management drugs can be divided into joint pain, neuropathic pain, cancer pain, chronic back pain, postoperative pain, migraine, fibromyalgia and other analgesic drugs. In 2015, opioid analgesics accounted for 49% of the major market for pain management drugs due to a significant increase in the number of patients with severe pain. According to statistics, 10%-40% of citizens in the United States suffer from different degrees of chronic pain. In addition, cancer patients, surgical drugs, and opioids have a very high market demand.
Opioids are a class of drugs similar to those found in opium poppy, including illicit drug heroin, synthetic opioids such as fentanyl, and painkillers that can be legally used by prescription, such as OxyContin (Oscardine) , Vicodin, cocaine, morphine and many other drugs.
In the treatment of chronic pain or severely ill patients, prescription opioids are often used to relieve the pain of the patient, but the use of such drugs will greatly increase the risk of opioid use disorders, resulting in addiction; use of opioids Excessive, it is easy to cause cardiac arrest, apnea and other fatal symptoms.
According to the US Department of Health and Human Services, more than 14,000 institutions in the United States are currently being investigated for the abuse of opioids. 116 Americans die every day from opioid abuse, and nearly 80% of heroin addicts use it before taking heroin. Prescription opioids.
Since the 1990s, the FDA has approved a series of opioids. In the case of OxyContin, OxyContin is an inexpensive painkiller with a chemical structure similar to heroin that produces an approximate morphine effect, but relative to the latter two. safer.
The pharmacology of OxyContin is to block the nerve pathway and block the transmission of pain to the brain. Excessive use will cause severe withdrawal reactions and cause drug dependence. Since 2000, the number of crimes caused by the abuse of OxyContin in the United States has soared, and OxyContin has also won the title of “Village Heroinâ€.
2. Opioid substitution for drug development
Previous reports from the arterial network showed that in the United States, the development of non-opioid painkillers has been more than 10 years old, but the results are not satisfactory. In the past five years, the so-called innovative painkillers approved by the FDA have been newly added to non-specialized drugs. So far, there is still no real innovative painkiller that can replace opioids, and there are no more than two innovative painkillers for new targets. (See: "The FDA's serial moves, digital health technology may become a new export of the opioid crisis.")
The reason is that the industry believes that there are mainly the following aspects:
First, technical limitations. Pain is a subjective feeling and a sign of disease, not the disease itself. In this respect, no biomarker can accurately monitor the enhancement or reduction of pain. On the other hand, in clinical trials of painkillers, patients will show a strong placebo effect, and the test data for the efficacy of new drugs is difficult to clean.
Second, regulatory restrictions. The regulatory framework for painkillers presents a plethora of clinical trial data requirements for innovators. Unlike opioids, which can target large areas of pain, new target analgesics are often targeted at a limited number of types of pain, such as pain caused by chemotherapy or pain caused by nerve compression. In order to meet regulatory requirements, innovators need to perform a large number of additional clinical trials.
Third, economic restrictions. Opioids are inexpensive and highly effective, and are highly recognized among doctors, patients, and health care payers. The price of innovative painkillers is relatively high, and the savings in medical costs need to be considered from the overall economic benefits of reducing side effects. The process of cultivating the market is long and the cost is not low.
3. The rise of the new pain management market
According to the British market consulting company Brisk Insights research report, in 2015, the global pain management drug and medical device market reached 37.8 billion US dollars, the compound annual growth rate is expected to be 4.3% from 2015 to 2022, and will reach 50.8 billion US dollars in 2022.
Innovative technologies and scientific research results are applied to the medical field, and the development of new pain management equipment is another major factor driving the market growth. In addition, the government's policy measures and economic investment have also promoted the development of the pain management industry.
According to research conducted by Hexun Finance, North America has the largest market in the pain management industry in terms of regional distribution, followed by Europe, Asia Pacific and other regions. The Asia Pacific market has the fastest growth rate. Regional market growth factors are mainly due to the increase in the number of patients with severe pain in the region and the huge government investment in hospitals. Another growth factor is companies' increased investment in research and development in new pain management drugs and medical devices.
However, strict drug and medical device regulatory measures in various countries and the lack of new pain management methods have become important factors restricting the development of the pain management market.
An unprecedented level of heat in this segment
Following the third article above, digital innovation in pain management seems to be a hot spot since 2018, and a new pain management market is emerging. The judgment given by mobihealth news is that “the number of news about chronic pain is more than usual, which seems to be a hot area of ​​digital healthâ€.
Through the reports of foreign media, many events have been disclosed in this field recently. In order to observe the development track in this field, we have lengthened the timeline and first sorted out a series of events related to pain innovation in the past two years abroad. The timeline is near To the far, it includes both the business activities of corporate acquisitions, the market behavior of innovative equipment through FDA certification, and the practice and application of large enterprises in this field.
August 16, 2018
WellBrain, a Bay Area meditation-based addiction prevention and chronic pain management platform, announced that it has acquired Mevoked, a behavioral analysis platform that currently provides digital procedures for managing perinatal and postnatal mental health.
Inspired by Tibetan monks' meditation practice, WellBrain's meditation platform is designed to prevent pain drug addiction by helping providers assess any mental illness or condition that may lead to addiction.
Based on the assessment, the software can be used to assign a customized pain management plan to the patient, who interacts with the platform using the iPad and the single-lead EEG headset provided by the doctor. At the same time, the doctor can view the data collected during the session to get more information about the condition.
August 16, 2018
Wearable device provider SPR Therapeutics announced on the 16th that the FDA has approved the launch of its new version of the percutaneous wearable pain management device.
June 5, 2018
Risalto Health, a New York-based musculoskeletal digital health platform, completed a $1.5 million seed round of financing for Health Catalyst Capital Management.
The platform serves as a third-party platform for consulting services that use AI to identify musculoskeletal health providers in a region, such as physiotherapists, doctors, and surgeons. Patients and employers are then linked to these providers based on big data needs to provide the most appropriate service provider for patients.
May 30, 2018
The road to addressing the crisis of opioid abuse through drug substitution is not going to work, and the FDA can only find another way. On May 30th, the FDA launched an innovation challenge to stimulate the development of digital health and diagnostic medical devices for pain and addiction. Innovators can apply for this challenge from June 1st to September 30th.
March 14, 2018
At last week's HIMSS, Samsung announced a partnership with Travelers Insurance, Cedars-Sinai Medical Center, Bayer and Applied VR to manage pain using virtual reality. Samsung and travelers will fund a study of 90 to 140 patients over a 16-month trial using technology from Samsung, Bayer and AppliedVR at the Cedars-Sinai Medical Center.
February 16, 2018
The Brigham and Women's Hospital in Boston is helping to conduct a study to determine if mHealth data collected on smartphones and wearables can be used to help treat patients with chronic pain.
The hospital is working with California-based digital analytics company Evidation Health, a company launched from the Stanford Health-GE Venture Incubator to capture "digital signals" from sensors in mobile devices and fitness bands. Researchers will examine whether these data are combined with other information such as weather, diet, genomic information, and medical data to determine when someone is in pain and to develop a treatment management plan.
Evidation Health contributed $1 million to the Digital Signal for Chronic Pain (DISCover project), which will recruit 10,000 participants, including 6,000 patients with chronic pain.
February 21, 2018
Behavioral Health Therapy App Manufacturer 2 Morrow announced its service to increase the digital chronic pain management program.
Much like the company's other products (for smoking, weight and stress) - the pain management program is based on Acceptance and Commitment Therapy (ACT), a clinically tested form of cognitive behavioral therapy that is strongly supported by the Clinical Psychology Society. The 2Morrow program, developed by the company in collaboration with Dr. Kevin Vowles, a behavioral scientist at the University of New Mexico, contains short lessons and exercises that teach users how to stay alive with chronic pain. The application-based application also allows the user to send a message to the actual pain management coach if there is a problem or requires specific assistance.
January 10, 2018
The Toronto-based startup ManagingLife has created an app that allows patients with chronic pain to track their symptoms. This week, the company announced the app, called Manage My Pain, which has been deployed in four chronic pain agencies in Ontario.
Innovative companies focus on nerve stimulation, large enterprises, health systems participate in cooperation
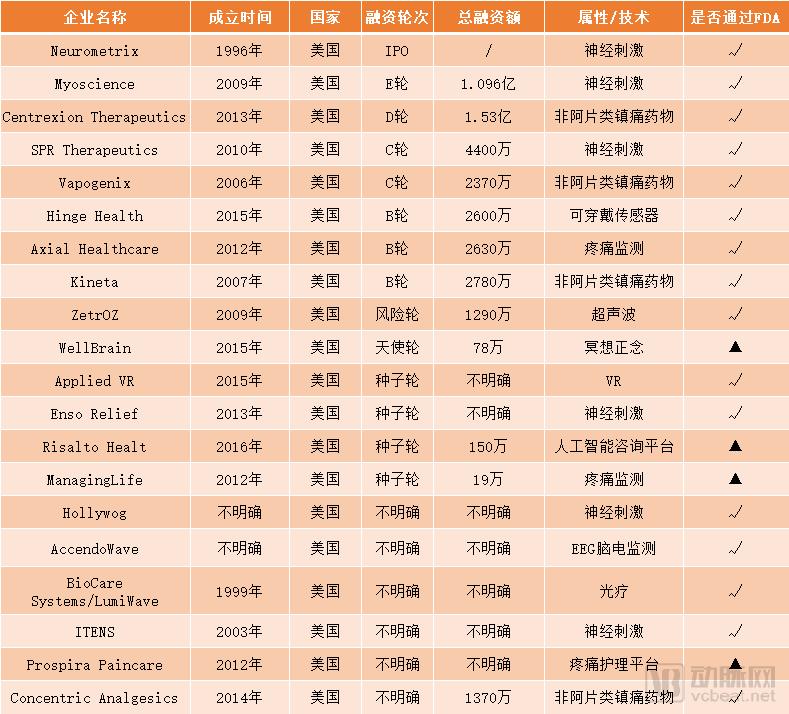
Note: In the column of whether or not to pass the FDA certification, “√†indicates that it has passed, “▲†indicates that it has not passed or is not required.
Foreign digital pain management companies (source: Crunchbase)
Although it is a very subdivided field, in terms of current market conditions, the pain management wearable space has a lot of competition for homogenized products.
Neurometrix's Quell iterates the latest version, allowing users to directly control pain management using the smartphone app and was approved by the FDA in November 2016.
SPR Therapeutics received $25 million in Series C financing for its nerve stimulation (PNS) system, which does not require implants or invasive procedures, but can be connected to the line with a diameter of only 0.2 mm through the skin.
In addition to startups, companies including electronics giants Samsung, Philips, and established equipment tycoon Medtronic are conducting innovative practices on pain management.
Philips introduced two new smart devices as early as 2014: PulseRelief and BlueTouch.
PulseRelief's function is to relieve muscle and bone pain by simply attaching the device to the affected area, stimulating the nerve by releasing current, blocking the painful nerve signals, and promoting the secretion of an analgesic endorphin to relieve pain.
BlueTouch is used to treat back muscle soreness, put the device on the back, blue LED lights stimulate the body to release more nitric oxide, promote blood circulation, and accelerate muscle self-healing.
Both devices can be controlled by iPhone or iPad, with different intensity levels or treatment types, such as PulseRelief, which allows users to control from their mobile phone, with 60 intensity levels to choose from. The treatment of pain through smart devices has revolutionized traditional analgesic medical devices.
In addition, Medtronic's implantable pain management device, Intellis, is also FDA-approved, and the device can be wirelessly programmed via a Samsung tablet.
The Intellis system is designed to treat chronic pain through nerve stimulation in the spinal cord. The introduction of the Intellis platform is not just a new device, but the Evolve workflow combines cutting-edge hardware with optimal treatment for personalization and long-term pain relief. In addition to providing nerve stimulation, the implant also monitors the user's activity, which can help the doctor determine the optimal treatment plan.
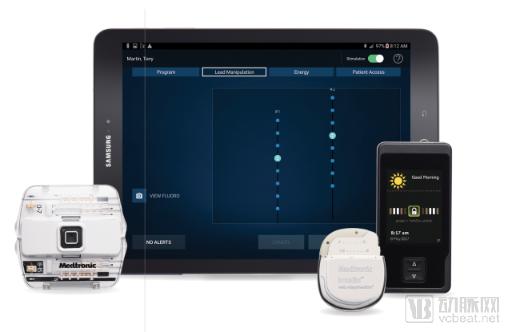
Intellis system (Source: mobihealthnews)
Purdue Pharma and GeisingerHealth system are also advancing research on chronic pain treatment provided by Apple Watch.
Many pharmaceutical companies are now turning to digital tools to improve drug adherence, increase clinical trials, or develop parallel therapies with digital healthcare companies. It is rare for pharmaceutical companies to actively stop people taking drugs, but this is the purpose of a new study launched by Purdue Pharmaceuticals Inc., a blockbuster and OzContin, an opioid that subsequently triggered a national crisis. Manufacturer of Oscardine).
Use VR, TENS, EEG monitoring and other techniques to relieve pain and measure pain
Pain management medical devices are classified according to product type, drug type and indications, and can be divided into: electrical stimulation equipment, radio frequency ablation equipment, analgesic pumps, nerve stimulation equipment (spinal cord stimulation, deep brain stimulation, vagus nerve excitation, phrenic nerve). Stimulation, etc.).
In 2014, nerve stimulation equipment dominated the market due to the successful use of spinal cord stimulation equipment and deep brain stimulation equipment for patients with chronic pain such as the back. Among the medical devices for different pains, neuropathic pain treatment devices account for the highest proportion, and the compound annual growth rate is expected to reach 6% in the future.
In addition to traditional medical devices, nerve-stimulated technology manages pain in 24% of innovative companies in arterial network statistics. Among them, the most mainstream is transcutaneous electrical nerve stimulation therapy.
TENS
Electrostimulation devices include transcutaneous electrical stimulation neurotherapy, neuromuscular electrical stimulation devices, other stimulation devices, etc. Among them, the most popular for innovative companies is transcutaneous electrical nerve stimulation therapy, namely TENS technology. Among the traditional medical devices, there is a type of device called "TENS pain treatment device" (or low frequency therapy device), which is a device that uses nerve electrical stimulation therapy, and has been popular in Europe and the United States for many years.
The medical community has generally believed that nerve electrical stimulation therapy has a certain clinical effect on relieving pain. Transcutaneous electrical nerve stimulation can send low-voltage electrical signals from the small device to the painful area through the mat attached to the skin, so that pain can be relieved in a short period of time, especially for various types of muscle pain. Although the researchers are not sure why it works, they believe it may disrupt the brain's nerve signals or stimulate "feeling good" endorphins, known as "the body's natural painkillers."
In theory, electrical impulses can prevent the brain from receiving pain signals and even promote the brain to produce natural endorphins, thereby inhibiting pain. Traditional TENS therapeutic devices have some drawbacks, and their actual effects are usually different from person to person. For example, some users may feel numb, which may be caused by incorrect device frequency setting.
VR
Researchers believe that VR can not only distract patients from acute pain, but also block their brain pain receptors, just like prescription opioids.
VR pain management research is favored by many large companies, Samsung, Travel Insurance, Bayer, AppliedVR in the Cedars-Sinai (Sinai Medical Center) pilot, hospital report feedback, in a small controlled study, VR can The patient's average self-reported pain score decreased from 5.4 to 4.1, while the 2D experience only reduced the score to 4.8.
Coincidentally, the experiment at the Sinai Medical Center is not the only investigation of VR's impact on the brain. For Dr. Brandon Birckhead, a radiation oncology resident at the University of Wisconsin Medical School and a VR researcher, some of the most exciting is the in-depth study of the role of virtual reality in experience cognition, or in other words, how avatars in VR might Will rewrite the relationship between the patient's brain and its motor system.
This is a 30-person study exploring the feasibility of this therapy, led by Dr. Kim Bullock, clinical associate professor of psychiatry and behavioral science at Stanford University, which is currently underway and is expected to be completed by the end of the year.
Wearable device monitoring
There is no hard and fast rule for measuring pain, and it may take a long time for the patient to describe his or her feelings to the doctor. Digital assessment of pain has also become one of the entry points for innovative companies.
Nanshan Hospital in Nevada recently completed a technology pilot that uses a Samsung tablet and a wearable EEG reader to assess patient pain and try to mitigate pain through distracting content.
The pilot, co-operated by technology company AccendoWave and Nevada Nanshan Hospital, is equipped with Samsung Galaxy tablets for nearly 1,000 emergency patients including adults and children, modified with Samsung Knox kit, and EEG reading headband (not Samsung Brand), from the photo it seems to be an Interaxon Muse headband. Tablets and headbands replace the hospital's normal system for reporting patient pain: a 10-point scale with smiles and frowns that represent different levels of pain.
Using EEG technology, the sensor is able to guess the patient's pain level and present it on the screen. The patient can then choose to agree or disagree to reach the level of pain sent to the doctor and nurse. Tablets also feature a range of video and music content, and then serve patients based on patient comfort and choice.
In addition to the above techniques, some of the latest high-tech methods to alleviate chronic pain include:
Radio waves: Radiofrequency ablation involves inserting a needle next to the nerve for pain and using the current generated by the radio wave to burn the nerve. This will short the pain signal and relieve pain for up to a year.
Nerve block: Using X-ray imaging, a pain medicine doctor can inject an anesthetic to stop or relieve pain and even prevent the development of chronic pain. The location of the injection depends on the source and type of pain. For example, arm or facial pain can be relieved by occluding the neck nerves. Relief may require a series of injections and repeated treatments.
Spinal cord stimulation: When other methods fail to relieve pain, pain medical experts may recommend spinal cord stimulation (SCS), which uses a pacemaker-like device to replace pain with a more tolerant sensation, usually a stinging or massage-like sensation . The doctor implanted the device into the lower back and attached it to the thin line inside the spinal canal. When the patient feels pain, they can use the remote control to send a signal to the painful area.
This technique can help treat back pain and leg nerve damage leading to neuropathic pain such as numbness and pain, which is common in people with diabetes. The new form of SCS shows hope for pain relief without tingling.
Pain Pump: A special pump can be implanted so that the patient can press the button and deliver painkillers to the spinal cord to relieve pain without the side effects of taking these drugs by oral administration. By directly controlling pain, patients can also get psychological stimulation. These spinal drug pumps are most commonly used by cancer patients, but are also used by patients with other types of pain who have side effects when taking oral medications.
Future solutions: One of the most promising areas of research is the role of stem cells in healthy tissues. Part of the pain is painful as the tissue has deteriorated. Stem cell therapy involves collecting stem cells from the patient's bone marrow and injecting them into areas such as the lower back, hoping that stem cells can build new healthy tissue and relieve pain.
Digital pain management typical enterprise
1. NeuroMetrix
Headquarters: Boston
Disclosure funds: IPO

The main product of NeuroMetrix in Boston, USA, is Quell, a pain relief device that primarily reduces pain in people with chronic pain disorders such as arthritis, frozen shoulder, and more.
The device is worn on the lower leg and works on the principle that Quell releases current to stimulate the sensory nerves, which then pulse the brain to signal the brain to produce a natural analgesic to relieve pain. Clinical trials have shown that the Quell Pain Relief Instrument is very effective for pain caused by painful diabetic neuropathy, fibromyalgia, sciatica, and osteoarthritis, and it works as soon as a quarter of an hour.
The Quell Pain Relief Device is currently certified by the US Food and Drug Administration and can be worn for extended periods of time with up to 40 hours of battery life. It can also be monitored for sleep through the iOS app and can reduce current intensity during sleep. When worn, it can relieve pain by 80%.
The device is currently priced at $249, but it is not suitable for the user. Patients with metal pacemakers, defibrillators, etc. implanted with metal and electronic therapy devices are prohibited from using the Quell pain mitigation device.
2. Myoscience
Headquarters: California
Disclosure funds: $109.6 million (round E)
Investors: Accuitive Medical Ventures, American Equities Overseas, De Novo Ventures, Medicis Capital, Saratoga Ventures
MyoScience provides focused cold therapy to address the pain caused by peripheral neuropathy. Its leading product is the iovera system.
The ioverao system is a non-opioid and non-systemic drug used to block pain signals from a variety of peripheral nerves. The iovera° system includes a small handheld device that uses a disposable tip to cool the focus to the target nerve throughout the body. It has proven to be a safe and effective method for the treatment of pain in independent, non-opioid drugs for the treatment of chronic, procedural and acute pain. Iovera° treatment provides immediate, long-lasting pain relief by blocking the ability of the nerve to transmit pain signals without affecting the structure of the target nerve. The system is currently FDA-approved to prevent pain, and it can also be used to relieve pain and symptoms associated with knee osteoarthritis for up to 90 days.
3. SPR Therapeutics
Headquarters: Beachwood, Ohio
Disclosure of funds: $43.3 million
Investor: Frontcourt Group
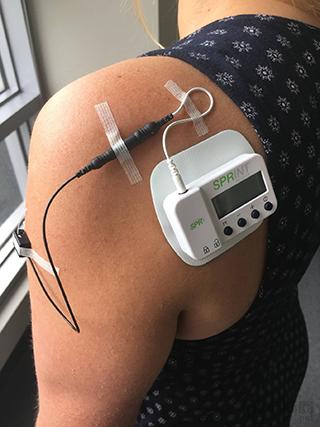
SPR Therapeutics products (Source: mobihealthnews)
SPR Therapeutics uses its peripheral nerve stimulation (PNS) platform technology to treat acute and chronic pain. SPR uses the FDA-approved Sprint Peripheral Stimulation (PNS) system as a non-narcotic alternative to chronic and acute pain relief. The wearable stimulator is connected by a wire that can pass through the skin with a diameter of only 0.2 mm, which relieves the patient's pain by activating the target nerve fibers.
On August 16, 2018, with the approval of the new 510(k), the Sprint Peripheral Stimulation (PNS) system is now available as a single-lead (Sprint endura) or dual-lead (Sprint extensa), which allows non-surgical placement of two The wires are connected to the wires of a single device. In addition, the updated platform now supports rechargeable batteries and Bluetooth-enabled controllers for patients to use.
4. Axial Healthcare
Headquarters: Nashville, Tennessee
Disclosure funds: $26.3 million
investor:. 406 Ventures, BlueCross BlueShield Venture Partners, Oak HC / FT Partners, Sandbox Industries
Axial Healthcare is a pain management solutions company with a platform that connects doctors, health insurance companies, pain clinics and patients. The company's four software products facilitate this and provide greater insight into the parties. Its products include:
axialAnalysis to measure patient risk, clinical outcomes, and performance of practitioners during pain episodes;
axialNetwork, used to measure pain management clinical outcomes, patient satisfaction, and seizure costs;
axisConnect, as a platform for patient participation, monitoring and clinical decision making;
The axialPatient and a patient-involved application called Empower help patients track their condition and manage side effects and treatments, including medications.
5. Hinge Health
Headquarters: San Francisco, California
Disclosure of funds: $11.3 million
Investors: Atomico, Eleven Two Capital, Jon Reynolds, The Vertical Group

Hinge Health's products (Source: Hinge Health's official website)
Hinge Health is an innovative company whose products include wearable devices and software platforms that provide patients with technologies to combat chronic back pain and joint pain. The specific product kit includes two sensory bandages with motion sensors and a tablet with pre-installed Hinge Health software for viewing rehabilitation videos, as well as stands, carrying cases and charging devices.
For chronic pain in the musculoskeletal, Hinge Health's data shows that it can cut costs by half. Using Hinge Health reduces pain by 70% while reducing surgery by 63%.
In terms of business model, Hinge Health is not directly oriented to C-side customers, but more to corporate employers, adopting the B2B2C model. Hinge Health is responsible for product development and production, but not directly to consumers, but to bring products to users through the health care plans of major companies.
China's painful diseases are still solved by hospitals, and innovative enterprises are scarce
Pain is actually a series of diseases, including acute pain, chronic pain and cancer pain, and sometimes even a combination of these diseases. The largest number of people in the country are suffering from muscle and bone connective tissue diseases, such as the most common cervical spondylosis, and the number of people is about 200 million. In addition to orthopedics and muscle diseases, pain may also occur for other reasons, such as metabolic problems such as surgery, nerve damage, and diabetes. Occasionally, the pain itself may even be a problem for no apparent reason.
In China's medical environment, the treatment and management of pain is still in the business model of solving most problems in hospitals. The treatment methods are usually surgery, minimally invasive surgery or physical therapy, drug therapy. Innovative pain management companies in the market are rare.
In the United States, Canada and other countries, pain is a specialized discipline, and there are specialized doctors and institutions to solve such problems, such as the pain clinic in the United States. In a previous interview, the reporter learned from the founder of Hepu Medical, Jingdong, that there were 157 certified anesthesia-based pain training bases and 97 certified pain specialist training bases in Canada in 2006. Thirty percent of the doctors who performed the pain treatment business received formal pain management training.
However, the composition of pain treatment in China is not standardized. It is mainly composed of Chinese medicine massage doctors, acupuncturists, anesthesiologists, orthopedic surgeons and psychiatrists. About 90,000 people have many irregularities in the diagnosis and treatment process, which makes China present. 70% of patients with chronic pain fail to receive timely and standardized treatment.
As the medical field learns more about the complexity of pain, it is even more important for doctors with specialized knowledge and skills to treat these diseases. Gain insight into the physiology of pain, assess the ability of patients with complex pain problems, understand specialized tests for diagnosing pain conditions, appropriate prescriptions for drugs for different pain problems, and skills to perform procedures (eg, nerve block, spinal injection) And other interventional techniques are part of the pain management specialist used to treat pain.
In public hospitals in China, 90% of pain tasks are carried by anesthesiologists. In hospitals, the establishment of pain departments is also a burden for anesthesiologists who are overworked. Therefore, even in hospitals, the pain department There are certain difficulties in setting up.
In terms of social capital participation, unlike the professional pain clinics or nursing institutions in the United States, the pain specialist clinics in China's pain management business are currently only Hepu Medical, and the representative pain doctors group has Chuanpai doctors group. In addition, Rehabilitation-based clinics or institutions also include pain management services, including clinics or outpatient clinics for sports rehabilitation and orthopedic rehabilitation. Representative enterprises include veteran sports rehabilitation clinic Hongdao Sports Rehabilitation Clinic, orthopedic rehabilitation clinic, excellent rehabilitation clinic, main attack The ridges of the spine rehabilitation are nearly perfect and the neck medical guards for cervical and lumbar problems.
The current specialist services involving pain diagnosis and treatment are still intertwined with rehabilitation, and the business lines of each company are crossed. The pain management innovation medical device market is almost in a blank state. The reporter only found that Xinyun Medical has previously released a smart cloud sticker for pain, and the company has now transformed into a provider of pain specialist medical joint solutions. In addition, enterprises and products related to wearable pain relief equipment include ipainfree produced by Jiangsu Aipeng Medical Technology Co., Ltd., Lefan Magic Sticker of Xiamen Mengfali, and LED wearable therapeutic instrument of Yijiantang.
In general, the service market for pain is still very immature, and enterprises are in the verification stage of business model. In terms of digital innovation of medical devices, it is difficult to compare with the development of foreign countries. In addition to the technical backwardness, there are also market education immature. From the pain clinic, which has already been popularized abroad, and the medical system based on the top three hospitals that are difficult to shake in the country, the importance of market education is far more than self-determination. The technology developed is much more difficult.
Carbomer is an acrylic resin obtained by cross-linking pentaerythritol with acrylic acid. It is a very important rheological regulator. After neutralization, carbomer is an excellent gel matrix, which has important applications such as thickening suspension. According to the dry product calculation, including carboxylic acid group (-cooh) should be 56.0 % ~ 68.0%.
Common carbopol series include: Carbopol 940, 941, 934, 1342, 980, ETD 2020, AQUA sf-1, Ultrez 21, Ultrez 20, etc.
Carbomer,Carbopol,Carbopol 940, Carbomer 940, Carbomer 980, acrylic acid
Xi'an Gawen Biotechnology Co., Ltd , https://www.ahualyn-bio.com
