Change "cold tumor" into "hot tumor", new drug reverses immunotherapy resistance
Change "cold tumor" into "hot tumor", new drug reverses immunotherapy resistance
April 18, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Today, Checkmate Pharmaceuticals announced at the 2018 annual meeting of the American Association for Cancer Research (AACR) the clinical phase 1b of the combination of CMP-001 developed by the company and Keytruda (pembrolizumab) from Merck (MSD) for advanced melanoma. Test Data. CMP-001 is an agonist of Toll-like receptor 9 (TLR9), and pembrolizumab is a PD-1 immunological checkpoint inhibitor. The results of the trial indicate that the combination of CMP-001 and pembrolizumab provides significant and sustained relief of tumors in patients who are resistant to single PD-1 inhibitor therapy.

The birth of immunological checkpoint inhibitors is a major breakthrough in the history of cancer treatment, allowing patients' own immune cells to recognize and kill tumors. However, in addition to the expression of a ligand for an immunological checkpoint receptor to inhibit T cell activation, there are other means for forming an immunosuppressive microenvironment around the tumor. One method is to recruit immature plasmacytoid dendritic cells (pDCs). Immature pDCs do not express type I interferons (IFNa), which support the function of immunosuppressive regulatory T cells (Tregs) and myeloid derived suppressor cells (MDSCs).
MAT-001, developed by Checkmate, binds to the TLR9 receptor and activates immature pDCs, allowing them to generate large amounts of IFNa, while increasing the expression of other costimulatory factors and presenting them to tumor antigens of T cells. These processes combine to form an effective anti-tumor T cell response.
As of March 27, 2018, a total of 85 patients with advanced melanoma received combination therapy with CMP-001 and pembrolizumab. CMP-001 is injected into one or more lesions on the patient in an intratumoral (IT) manner. The researchers evaluated the response of all targeted and untargeted lesions using the solid tumor efficacy evaluation standard version 1.1 (RECIST 1.1).
The results of the trial indicate that the combination of CMP-001 and pembrolizumab is capable of eliciting significant and long-lasting systemic tumor shrinkage in patients who have received both therapies on average and whose symptoms continue to worsen. Not only did the skin lesions that received the injection be relieved, but the skin lesions that were not injected and the metastases in the lymph nodes, liver, and spleen were also significantly reduced. In 69 intent-to-treat (ITT) patients, the objective response rate (ORR) was 22%, including 2 complete remissions and 13 partial remissions. Of the patients who achieved remission, 6 patients had a duration of more than 6 months, and 2 of them had a duration of more than 84 weeks.
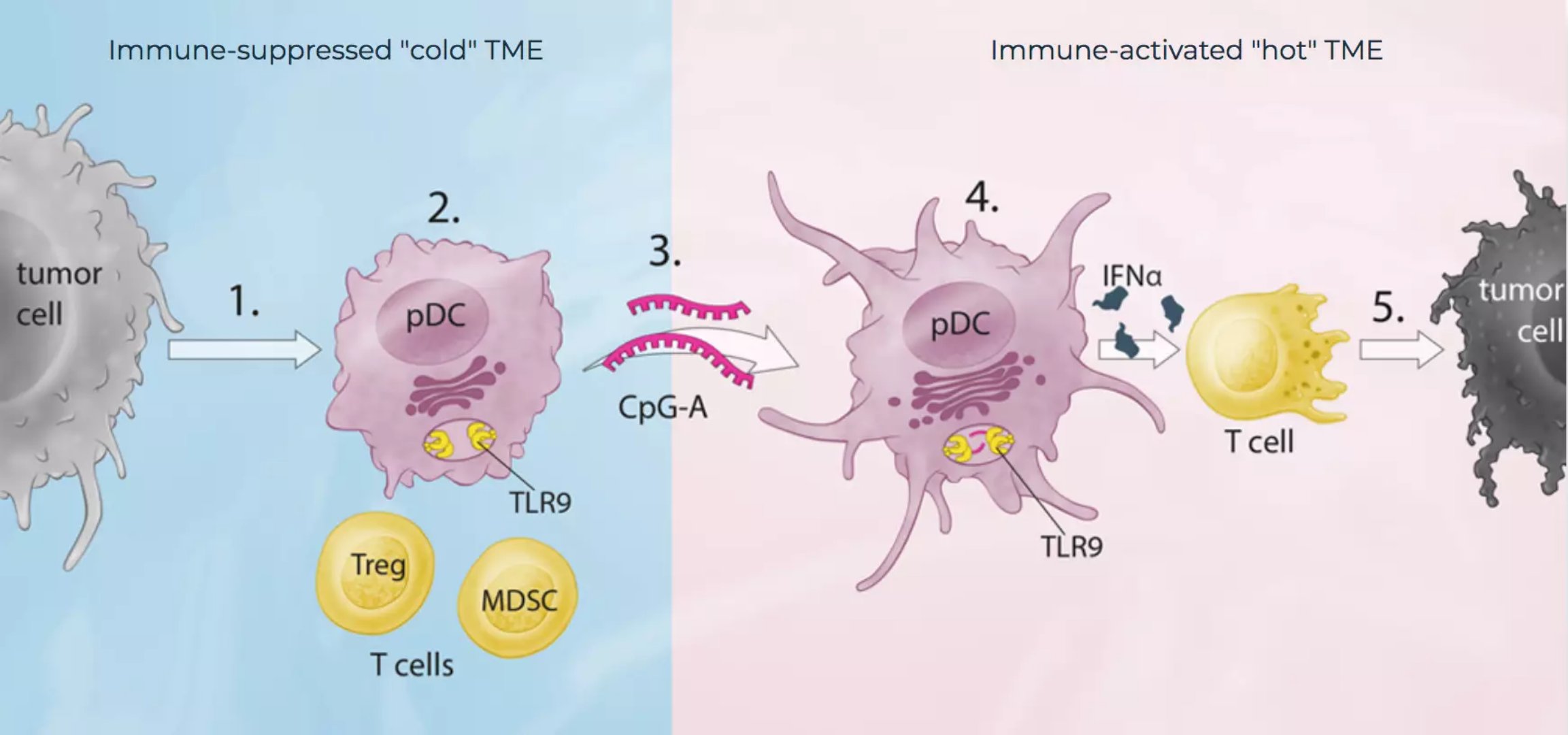
â–² CMP-001 (CpG-A) mechanism of action (Source: Checkmate Pharmaceuticals official website)
The detection of the immune microenvironment near the tumor confirmed the effect of CMP-001 on the activation of pDC surface TLR9 near the tumor, including:
1. The level of CXCL10 chemokine induced by interferon in serum increased by an average of 5.9 times.
2. Increased expression of CD8-positive T cells and PD-L1 at the lesion
3. RNAseq analysis of gene expression profiles at the lesions showed that gene expression exhibited characteristics associated with T cell inflammatory responses and PD-1 responses.
4. Patients with poor response to combination therapy have a small number of pDCs in the tumor before treatment, while patients responding to combination therapy have 10 times more pDC before treatment.
“The abscopal effect observed in patients is a classic hallmark of successful intratumoral immunotherapy,†said Dr. Mohammed Milhem, a clinical professor at the University of Iowa, who is responsible for the clinical trial. "In this group of patients, the remission rate of pembrolizumab monotherapy is almost impossible to exceed 7%. If these results are validated, this combination may provide an effective treatment option for patients with advanced melanoma who are resistant to pembrolizumab."
Dr. Art Krieg, founder and CEO of Checkmate, added: "The anti-cancer mechanism of CMP-001 is not limited to melanoma, it may be used to treat most tumor types, including those that previously did not respond to PD-1 therapy. The tumor."
We expect this new treatment to bring new hope to patients who have not responded well to single immunotherapy.
Reference materials:
[1] Checkmate Pharmaceuticals Presents Clinical Data at the 2018 American Association for Cancer Research (AACR) Annual Meeting
[2] Checkmat Pharmaceuticals official website
Automatic Massage Collapsible Foot Spa Massager
Auto Massage Foldable Foot Spa Massager is a device that provides relaxing and rejuvenating foot massage. It has a collapsible design for easy storage and transport. The massager has multiple massage rollers that target specific pressure points on your feet to deliver a deep tissue massage that helps relieve tension and improve circulation. It also has a heating function that can help soothe sore muscles and promote relaxation. The massager is easy to use and can be controlled with the push of a button. It's a great way to pamper yourself after a long day on your feet or to relieve foot pain and discomfort.
Automatic Massage Collapsible Foot Spa Massager,Folding Foot Bath Machine,Foot Spa,Massager Folding Foot Spa
Huaian Mimir Electric Appliance Co., LTD , https://www.mmfootbath.com
