Cell therapy for myocardial ischemia, FDA release key clinical trials
Cell therapy for myocardial ischemia, FDA release key clinical trials
February 01, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];BioCardia has announced that the FDA has approved its CardiAMP cell therapy for clinical trial equipment exemption (IDE) for chronic myocardial ischemia (CMI), enabling it to begin critical clinical trials using CardiAMP cell therapy for refractory angina (RA).

In the United States, an estimated 600,000 to 1.8 million patients have refractory angina, and there are approximately 75,000 new cases each year. Despite advances in revascularization techniques, there are still more and more patients with chronic refractory angina who are unable to undergo further revascularization, severely limiting the improvement of symptoms. These patients have poor health, significantly impaired quality of life, and suffer psychological distress, increasing the burden on the medical system. Current treatments are limited and the relief of angina is not significant.
CardiAMP® Cell Therapy is BioCardia's drug candidate for biotherapeutics in clinical development. The therapy uses an individualized minimally invasive method to utilize the patient's autologous bone marrow-derived cells for cell transplantation through a cardiac catheter for the treatment of cardiovascular disease. Bone marrow-derived cells are evaluated and quantified using patented biomarkers during treatment and then cell transplanted using their HelixTM endocardial delivery system. The therapy is designed to stimulate the body's natural healing response.
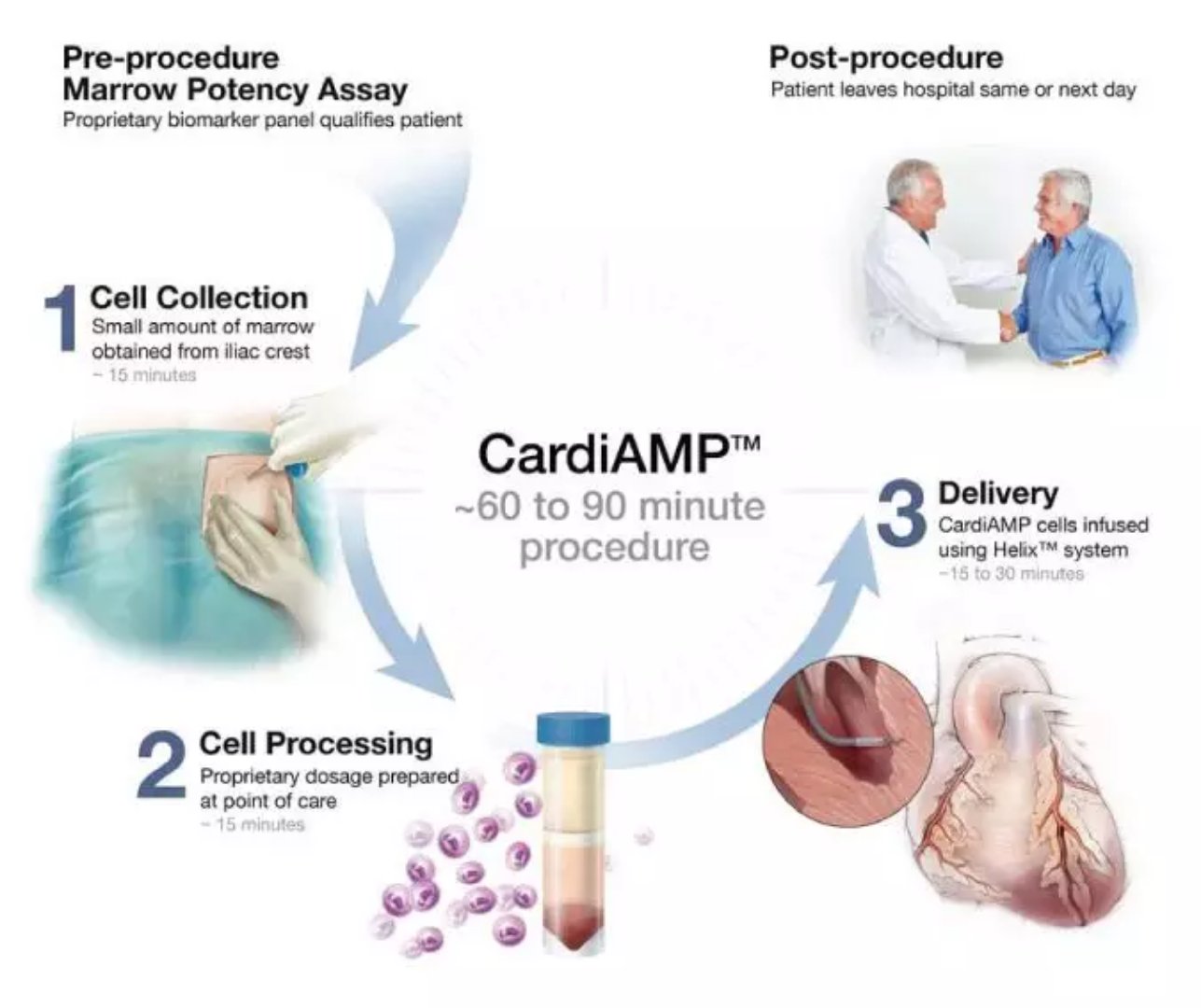
▲CardiAMP® Cell Therapy uses individualized minimally invasive methods to utilize patients with autologous bone marrow-derived cells for cell transplantation via cardiac catheterization (Photo: BioCardia)
The CardiAMP Chronic Myocardial Ischemia (CMI) trial will be a prospective, multicenter, randomized, sham-operated, double-blind, critical trial of patients and evaluators to validate CardiAMP research cell therapy in patients with CMI and RA Safety and effectiveness. The trial has been approved to enroll 343 patients in up to 40 US centers. This test may provide a functionally valid identification sufficient for product registration without a second confirmation test.
This clinical trial of CMI and RA for CardiAMP Research Cell Therapy is the second clinical trial following FDA approval of this therapy for critical clinical trials of ischemic heart failure.
Dr. Peter Altman, CEO of BioCardia, said: "The approval of the CardiAMP CMI trial by the FDA is another important milestone for the company. It marks a step towards a new therapeutic strategy that may be for patients with refractory angina. There is tremendous benefit. The approval of the second key trial of CardiAMP further supports BioCardia's leadership in the treatment of heart disease."
In the era of rapid development of cell therapy, we expect more effective cell therapy to alleviate the pain of heart disease patients.
Reference materials:
[1] BioCardia website
[2] CardiAMP Cell Therapy Receives FDA Approval for Pivotal Trial in Chronic Myocardial Ischemia
Greenhouse Plastic Plastic Clamps
Greenhouse Plastic Plastic Clamps,Greenhouse Structure Connecting Clamps,Agricultural Greenhouses Clamps,Plastic Clamps
JIANGSU SKYPLAN GREENHOUSE TECHNOLOGY CO.,LTD , https://www.alibabagreenhouse.com
