Medical Network April 15th In 2018, multinational pharmaceutical companies listed 44 new drugs in China, accounting for 77% of all approved new drugs. Among them, Gilead listed the most powerful drugs, the largest number of BI listings, Sanofi has a special liking for rare diseases, and "global big pharmaceuticals" Pfizer is the most frustrated. In the past two years, only two new drugs were listed in China. .
Recently, IDEA Pharmaceuticals released the 9th pharmaceutical company's annual innovation index (PII), in which Gilead first ranked the top of the industry innovation, followed by Aibowei, Eli Lilly, Pfizer, Merck, Sanofi, Novo Nordisk Roche, Novartis and GlaxoSmithKline. This ranking reflects the ability of pharmaceutical companies to bring products from the clinical phase 1 and phase 2 trials to the market and successfully commercialize them.
In China, with the continuous reform of China's new drug review and approval and clinical trial reform, the research, access and commercialization capabilities of multinational pharmaceutical companies' innovative drugs in China have increasingly become an important competitive factor in China. In particular, after the “4+7 quantity procurement â€, the high-growth model of patent expiration of multinational pharmaceutical companies will no longer exist, which further confirms this strategy. In the future, multinational pharmaceutical companies will also be innovative drugs in China!
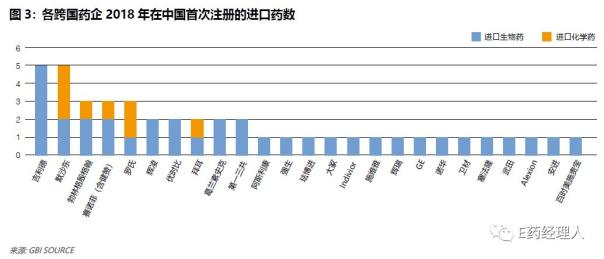
In 2018, the Chinese market was approved to list 57 new drugs, which is close to the FDA's record-breaking 59 new drugs in quantity, reflecting the fact that NMPA's review and approval efficiency is comparable. Among them, 33 imported chemical drugs and 11 imported biological drugs, and multinational pharmaceutical companies listed 44 new drugs in China, accounting for 77% of all approved new drugs. The continuous increase in the number of approved companies also reflects the efforts of multinational companies to expand their product lines.
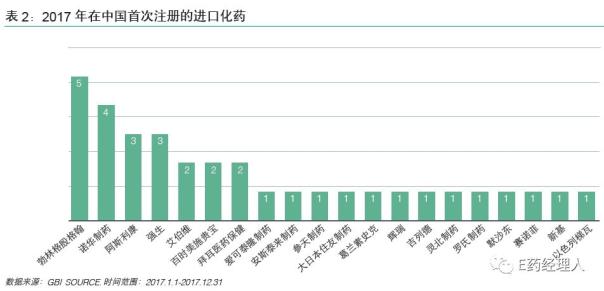
Among them, the top five pharmaceutical companies that listed the most new drugs in China in 2018 were Gilead, Merck, Boehringer Ingelheim, Sanofi and Roche. This is slightly different from the 2017 ranking: in 2017, the top five pharmaceutical companies that listed the most new drugs in China were Boehringer Ingelheim, Novartis, AstraZeneca, Johnson & Johnson, Abbott/Bournemouth Squibb/Bayer.
What are the innovative medicines of these multinational pharmaceutical giants in China? Who has the most listings? Who is the fastest? Who is wrong with the tricks?
01 , Gilead: Rapid expansion of the hepatitis market
In 2018, Gilead listed 5 new drugs on the Chinese market, and in 2017, 6 innovative drugs were approved for listing in 15 months, making it the biggest winner of the new drug approved by multinational pharmaceutical companies in China in 2018. .
This American company, which has only fully entered the Chinese market in 2016, clearly sees new drugs as the focus of its efforts in the Chinese market. The five innovative drugs approved in 2018 are rooted in the hepatitis and HIV/AIDS markets and have created multiple “firsts†in China.
In May 2018, following the listing of the heavyweight hepatitis C drug Sohuadi in China, Gilead's new heavy drug, Bundongsha (soprevovir/Vipathavir), was approved for the treatment of genes 1-6 in China. Adults with chronic hepatitis C virus (HCV) have also become China's first approved pan-genotype HCV single tablet regimen (STR).
Five months after the approval, Bundongsha was selected in the National Essential Drugs List (2018 edition) in October of that year, and it is very likely that medical insurance will be included in the new round of medical insurance catalog adjustment.
In August 2018, JF Kang (Ai Kao Bing Tablet) was approved for the treatment of HIV-1 infection in China, becoming the first approved single agent in China containing TAF/FTC for the treatment of HIV infection in adult and adolescents. Tablet program. Compared with the multi-tablet program, "all in one" and "one tablet a day" can better improve patient medication compliance. Globally, JF Kang brought more than $3.6 billion in sales to Gilead in 2017, an increase of 148% compared to 2016's $1.484 billion.
In November 2018, Gilead's three consecutive new drugs were approved in China, namely, Wei Lide, which treats chronic hepatitis B, Da Ke, who treats HIV-1 infection, and Xia Fanning, who treats type 1-6 chronic hepatitis C.
Wei Lide is the first new oral drug approved for the treatment of hepatitis B in the Chinese market in the past 10 years. After being approved, it is known as the “most powerful†hepatitis B drug. Verid has an antiviral effect similar to Gilead's tenofovir disoproxil fumarate (TDF), but at a dose of only one tenth of the latter.
02 , BI : 8 new drugs listed in China in 2 years
In the past two years, Boehringer Ingelheim (BI) has listed the largest number of innovative drugs in China, and it has gained a lot. Among them, five innovative drugs were listed in 2017, and one innovative small molecule drug and two bio-innovative drugs were also acquired in 2018.
Among them, the treatment of Odatrol aerosol and Tiotropium bromide/Odatrol inhalation spray is the latest generation of therapy, which has been listed abroad for more than 3 years and has sufficient clinical evidence. The launch of two new drugs has dramatically increased the clinical treatment options for COPD, and it is also expected to change the current status of the “one big one†in the asthma and COPD treatment market.
In 2017, BI used for the treatment of hypertension with telmisartan and amlodipine (double plus), for the treatment of type 2 diabetes, engliflozin (Ou Tangjing) and linagliptin metformin (Ou Shuangning), treatment Nidanibu (Vegatte) for pulmonary fibrosis and afatinib (Gitari) for non-small cell lung cancer have been approved in series, which not only greatly enriched the innovative drug pipeline, but also made Boehringer Ingelheim In China, it has expanded into cardiovascular, metabolic, oncology, respiratory, central nervous system, immunology and other diseases.
03 , Pfizer: Frustration of "Global Pharmaceuticals"
Compared with other multinational pharmaceutical companies, Pfizer, the "big global pharmaceutical company", has been slightly frustrated in the listing of innovative drugs in China in the past two years. Recently, at the 4th China Medical Affairs Conference (CMAC) held in Shanghai, Pfizer China Chief Medical Officer Gu Chengming publicly ridiculed: "We have not had much pressure in the past two years because we have no new drugs in China."
Although the number is small, in fact, Pfizer has been approved for a new drug in China in the past two years. In 2017, the oral JAK inhibitor tofatib, which is used to treat rheumatoid arthritis, was applied for in China. Pipercilili capsules (Aiboxin) for breast cancer treatment were approved in 2018.
It is worth mentioning that Tofatib, approved in 2017, is the first JAK inhibitor approved for the treatment of rheumatoid arthritis patients and has been approved in more than 50 countries around the world. Pipersulfil, approved in China in July 2018, is the world's first selective inhibitor of cyclin-dependent kinase (CDK) 4/6. In 2013, the US Food and Drug Administration (FDA) approved Aiboxin as a breakthrough new drug for the treatment of advanced breast cancer. In 2015, the FDA approved Aiboxin for rapid breast cancer treatment for advanced breast cancer. Based on this, the National Comprehensive Cancer Network (NCCN) guidelines recommend Aiboxin combined with aromatase inhibitors as a first-line treatment for HR+/HER2-advanced or metastatic breast cancer.
In the global market, the second year of Aibo's new listing exceeded $2 billion, and in 2017 it reached $3.5 billion, with a growth rate of over 117%. However, it is currently priced higher in China, with a price of 29,800 yuan per box and 7 boxes for half a year, or 208,600 yuan.
But this good new drug is not a good day in China. In November 2018, CPM data showed that the application of the generic drug of Pibsilili capsule submitted by Qilu Pharmaceutical was changed to the acceptance status. In addition, 13 companies including Haosen, Hengrui, Haizheng and Zhengda Tianqing were carrying out the Pibsicil capsule. Clinical Trials. Although Aiboxin is still in the patent period, it is foreseeable that this product will enter a fierce competition soon. Pfizer will also be under greater pressure.
On November 21, 2018, Pfizer's Pipersil capsule appeared in the catalogue of the Shenzhen Major Medical Supplementary Medical Insurance. Under the pressure of generic drugs, Pfizer may seek to cut prices or enter the medical insurance channel to seize the market in advance.
04 , Sanofi: Love the rare disease medicine
Sanofi listed three new drugs in China in 2018, a slight increase compared to a new drug in 2017. Two of the three new drugs are rare drugs.
China is gradually raising its focus on the treatment of rare diseases. In May 2018, the five ministries jointly announced the "First Uncommon Diseases Catalogue" to provide strong policy support for the diagnosis and treatment of rare diseases. On March 28, 2019, CDE announced the second batch of clinically urgent new drugs, including 14 rare diseases.
In July 2018, Sanofi's terifluamine tablet Obajie was approved for the treatment of relapsing multiple sclerosis (MS) in China, which is currently the first approved treatment for multiple sclerosis in China. Oral disease correction treatment drugs. Multiple sclerosis has been included in China's "First Uncommon Diseases Catalogue". From approval to drug delivery, Oberaje has only spent 58 days, setting the “fastest speed†for the recent introduction of innovative drugs for rare diseases in China.
In December 2018, Sanofi's rare disease treatment drug, Biling (Puleshafu Injection), was approved in China. As a new generation of hematopoietic stem cell mobilization mobilizer, valproate can significantly improve the success rate of hematopoietic stem cell collection, so that patients can get more cure opportunities through transplantation.
Since the acquisition of the American rare disease giant Genzyme, Sanofi has a special liking for rare diseases, and deep ploughing in the field of hemophilia and rare diseases. Globally, Sanofi acquired the Belgian pharmaceutical company Ablynx in 2018, which is a new drug for the treatment of rare blood coagulation disorders. At the beginning of 2018, Sanofi acquired Bioverativ for $11.6 billion. This drug company specializes in hemophilia. The two acquisitions cost Sanofi $16.4 billion.
Recently, Shanxi Province has also decided to include Sanofi's two rare diseases, the special medicine for treating Gaucher disease, and the special medicine for treating Pompe's disease, and to include the scope of major illness insurance and determine medical insurance. Payment standard . Next, the policy will provide corresponding support for rare disease drugs in various aspects such as review, access, medical insurance, and pricing. Rare diseases may also become the cradle of "blockbuster" in China.
Recently, Sanofi, executive vice president of Sanofi, global leader of generic drugs and emerging markets, also revealed in an interview that rare diseases will become an important part of Sanofi’s China strategy. To this end, Sanofi will also be in China and Emerging markets have formed a new business unit.
05, Merck: PD-1 first boarded China, nine-month HPV vaccine for 8 days
In 2018, the Chinese market is especially important for Merck. In the year, a total of five innovative drugs were approved in China, including the heavy PD-1 drug cerida and the “eight-day trial†nine-valent HPV vaccine. In the second quarter of 2018, Merck achieved a 26% year-on-year growth, and the Asia Pacific region is considered one of the regions with the strongest growth drivers.
Just a few weeks away from BMS's PD-1 drug Odivo, in July 2018, Merck's PD-1 drug, Ruida, was approved in China for the treatment of melanoma. Correda's global sales in 2012 were US$7.171 billion, and it also grew strongly in the Chinese market. It is reported that sales in the four months exceeded 500 million yuan. In early April 2019, K medicine was officially approved in China for first-line treatment of combined chemotherapy for non-small cell lung cancer. Globally, more than 60% of K medicine's income comes from lung cancer indications, which shows that it will continue to grow rapidly in the Chinese market.
After the approval of the nine-priced HPV vaccine in China, the demand exploded. Its exclusive dealer in China Zhi Fei biological November 2018 announcement that the future HPV vaccine purchase amount will increase significantly, which in 2019 amounted to 5.507 billion yuan purchase in 2020 of 83.3 billion yuan in 2021 to 4.165 billion yuan . They are 7.4 times, 11.2 times, and 5.6 times of 2017 purchases, respectively. The hot sale of HPV vaccine has greatly increased the performance of Merck. Merck's semi-annual report in China showed that sales in China in the first half of this year exceeded 6.557 billion yuan, an increase of 33.15% compared with last year's 4.924 billion yuan.
06 , Roche: I hope that innovative medicines will enter medical insurance.
In 2018, Roche listed three new drugs in China, including the new drug Aplaceinib and two bio-innovative drugs, Patuzumab and Emmet Salimizumab.
In August 2018, aletinib (Anshengsha) was approved in China for the treatment of ALK-positive locally advanced or metastatic non-small cell lung cancer. It is currently an ALK inhibitor of Best in class. On December 11, 2015, aletinib was first approved by the FDA. On November 6, 2017, the FDA approved alittinib as the first-line drug for patients with ALK+NSCLC. Basically realize the simultaneous listing of China and the United States. Globally, Aletinib's market share expansion is very strong, with sales of 362 million Swiss francs (+101%) in 2017 and sales of 279 million (+91%) in 2018H1.
In December 2018, Roche's innovative targeted drug, Patrice (Pertuzumab), was approved in China for the combination of trastuzumab and chemotherapy for patients with HER-2 positive early breast cancer who have a high risk of recurrence. Auxiliary treatment. Pertuzumab was developed by Roche's subsidiary Genentech and was first approved in 2012. Herceptin's dual-target treatment program has been recommended in several global guidelines and is approved in more than 75 countries worldwide. Paget has also been included in health insurance in 44 countries around the world.
Emeralduxumab is an innovative drug that has been included in the priority review process as a rare disease treatment and clinically urgently needed variety. It was developed by Roche's Japan-based Chinese and foreign pharmaceuticals for patients with hemophilia A with congenital coagulation factor deficiency. Conventional preventive treatment.
Roche's four targeted drugs trastuzumab, rituximab, bevacizumab, and erlotinib entered the medical insurance catalogue through negotiations in 2017, reducing prices by 50%-70%, two months later Medical insurance reimbursement quickly landed. According to E-pharmaceutical managers, Roche China’s sales in 2018 soared by 75% and sales increased by 28%. Zhou Hong, head of Roche China, recently said in an interview with the E-pharmaceutical manager that in 2019, he hopes to include all three new drugs approved in 2018.
Viral Transportation Medium Tube,Sample Collection Tubes,Transport Nasal Swab With Tube,Virus Sampling Tube Nasopharyngeal Swab